VP Ma’ruf Amin Receives First COVID-19 Jab
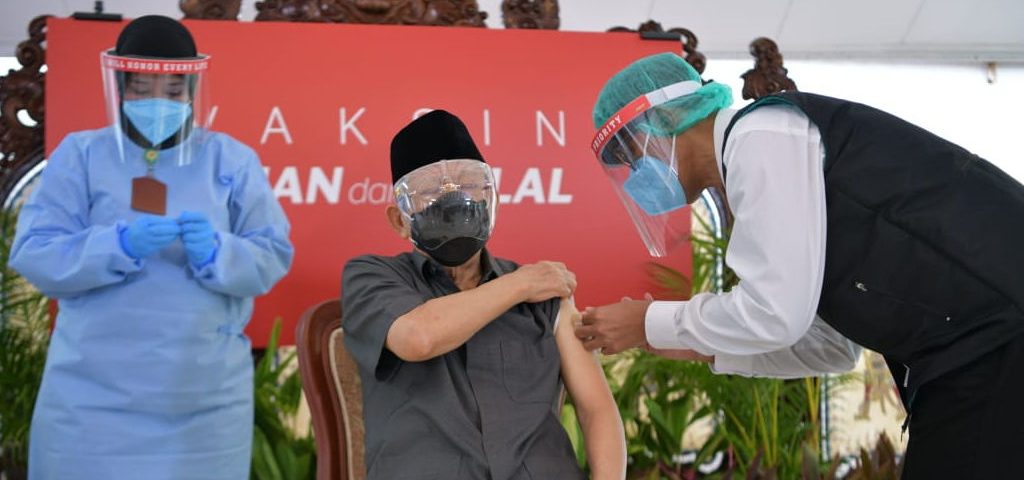
Vice President Ma’ruf Amin receives his first jab of COVID-19 vaccine, Wednesday (17/2), in Jakarta. (Photo by: Vice President Secretariat)
Vice President Ma’ruf Amin received his first jab of Sinovac’s COVID-19 vaccine on Wednesday (17/2).
The Vice President who is currently 77 years old is vaccinated in the vaccination batch two targeted to 21.5 million senior citizens aged 60 and above. In the first batch that commenced on 13 January 2021, the Government has rolled out vaccines to 1.46 million medical workers.
Participation of Ma’ruf Amin in the vaccination program is expected to ensure the safety of vaccine for use on senior citizens to the public.
Earlier this month, the Indonesian Food and Drug Monitoring Agency (BPOM) has issued Emergency Use Authorization (EUA) for Sinovac COVID-19 vaccine for use on senior citizens. They will receive two doses of vaccine in 28 days interval.
In the meantime, BPOM Head Penny Lukito said that the EUA is issued after detailed discussion between the BPOM, the National Commission for Drug Evaluation, Indonesian Technical Advisory Group on Immunization (ITAGI), allergy and immunology specialists, and geriatric specialists on the results of the Sinovac’s CoronaVac vaccine clinical trials in China and Brazil involving groups aged 60 and above.
Penny said that previously the BPOM has monitored and obtained data on the first and second clinical trials of the vaccine in China and the third clinical trial in Brazil. The approval was given after the BPOM examined clinical trials data of both countries.
Based on clinical trials in the first and second phases in China involving 400 elderly subjects, they showed strong immunogenicity and seroconversion rate of 97.96 percent 28 days after the second jab. The trials also showed that the CoronaVac vaccine brought no serious side effects.
“Meanwhile, the third phase of clinical trials conducted in Brazil by involving 600 elderly subjects showed that the vaccine is safe for use on senior citizens aged 60 and above, no serious side effects have been reported,” she said.
Data from Ministry of Health reported that senior citizens contributed 10 percent of the total COVID-19 cases in Indonesia and more than 50 percent of COVID-19 death occurred in patients aged 60 and above. It indicated that they are at the high risk from COVID-19.
For that reason, the Government has made vaccination for senior citizens a priority since they will most likely develop clinical deterioration following infection from COVID-19. (VICE PRESIDENT SECRETARIAT/ UN) (RAS/MUR)







